Previous studies looked at epigenetic mechanisms about macrophages and causes of immuno-metabolic diseases, like atherosclerosis and type 2 diabetes.
GPS2, a part of a fundamental gene-repressing complex, was found to be downregulated in monocytes and macrophages of humans with obesity and type 2 diabetes. This likely affects how inflammatory genes express, as the involvement of gene expression repressors remain ambiguous.
Read the original publication of this study here: The corepressors GPS2 and SMRT control enhancer and silencer remodeling via eRNA transcription during inflammatory activation of macrophages
This study used an in vitro macrophage model to investigate chromatin structure and histone modifications, transcription factor and coregulator dynamics, and enhancer RNA transcription along with gene expression, in response to pro-inflammatory signaling.
The corepressors GPS2 and SMRT control enhancer and silencer remodeling via eRNA transcription during inflammatory activation of macrophages
In this new study, Eckardt Treuter et al. looked to answer the questions of how do non-coding DNA elements regulate gene expression, what are the critical components involved, and can we therapeutically target them?
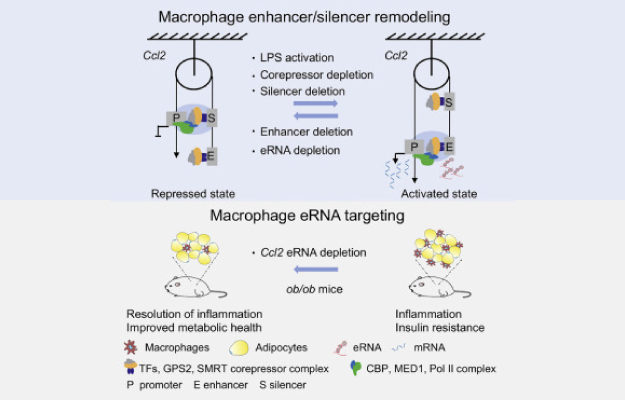
The researchers investigated how gene expression suppressors (coregulators) modulate enhancers and silencers during inflammatory macrophage activation.
Using inflammatory macrophage activation as a model, researchers investigated a corepressor complex containing GPS2 and SMRT, and how it encoded related signalling proteins.
The study of transcriptional interference, genome editing, and cistrome analysis
highlighted the dual ability of apparently related enhancer and silencer elements controlling Ccl2 transcription in seemingly opposite ways.
By reducing an eRNA of the critical Ccl2 enhancer using a modified short nucleic acid, the scientists could reduce the macrophage expression of CcL2 (aka MCP-1, a major inflammatory chemokine) in mice and therefore reduce metaflammation.
Thus, the identified corepressor-eRNA-chemokine pathway operates in vivo and suggests therapeutic opportunities by targeting eRNAs in immuno-metabolic diseases.
Takeaways:
- Corepressors are necessary for the signal-dependent chromatin remodeling within defined 3-D structures.
- The research sheds new light on enhancer-transcribed eRNAs having a more significant functioning role than previously thought.
- Macrophage eRNAs can be selectively targeted to reduce inflammation and thereby to improve metabolic health in obese mice.
- Further studies on combating immuno-metabolic diseases should be carried out.
You can read the original publication of this study here: The corepressors GPS2 and SMRT control enhancer and silencer remodeling via eRNA transcription during inflammatory activation of macrophages