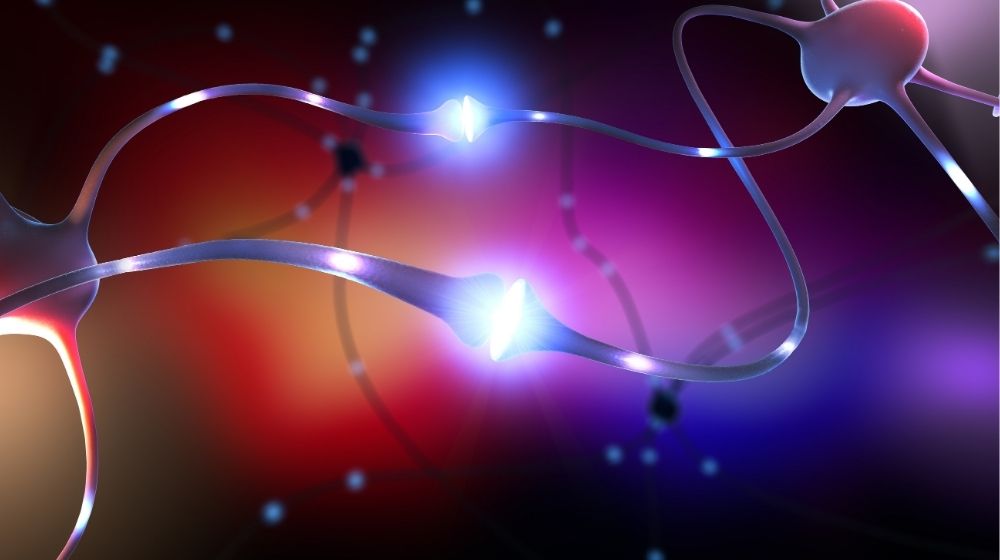
Neurotransmitters are the molecules that carry signals between neurons. Signaling between neurons enables us to learn, think, and experience mood changes. When signaling malfunctions, it may cause mental illness and cognitive difficulties.
The best-known examples are serotonin and dopamine, and both are involved in psychological illnesses, such as anxiety, addiction, and depression.
Serotonin regulates mood, and too little may contribute to depression. Doctors often prescribe selective serotonin reuptake inhibitors (SSRI) as treatment. It’s thought to increase serotonin levels and thus, improve communication between neurons controlling mood. In theory, it makes sense. But it doesn’t explain why it takes a month or more to reduce symptoms.
The brain’s reward center produces dopamine in response to something pleasant, such as a nice meal or winning a bet. Many addictive drugs increase dopamine levels, which creates a sense of euphoria. The chemically induced reward then initiates a craving for more. If the reward circuitry becomes damaged, it may cause depression, which could explain why someone with depression may self-medicate with substances that boost dopamine.
Researchers at the Icahn School of Medicine at Mount Sinai recently discovered serotonin also acts as a chemical marker. It binds to H3, a histone that regulates the genes in charge of turning stem cells into serotonin neurons.
Read the original publication of this study here: Dopaminylation of histone H3 in ventral tegmental area regulates cocaine seeking
This latest study aimed to examine if dopamine might act in a similar way to seratonin, regulating the genes involved in drug addiction and withdrawal.

Dopaminylation of histone H3 in ventral tegmental area regulates cocaine seeking
Scientists studied the postmortem brains of cocaine users. They found lower amounts of dopaminylation of H3 in the dopamine neuron clusters in the ventral tegmental area (VTA) of the brain. This brain region is known to be relevant in addiction.
Next, the team studied rats before and after ten days of self-administered cocaine. And like the human brains, dopaminylation of H3 was lower in the rats’ VTA. Furthermore, a month after withdrawing the rats from cocaine, the dopaminylation of H3 was much higher than the controls.
The increase may be crucial in controlling gene expression, rewiring the brain’s reward center, and generating cravings during drug withdrawal.
With long-term cocaine use, modified neural circuits need a steady supply of the drug to function normally. It requires activating some genes and deactivating others to make the proteins for those changes. In other words, dopamine acting on H3 is the driving force behind the epigenetic mechanism, not DNA sequence alterations.
Cocaine withdrawal is associated with changes in gene expression that rewire neural circuits and alter synaptic connections. But in rats genetically modified to inhibit dopaminylation, changes were suppressed. Moreover, these rats had lower neural impulse firing in VTA neurons and released less dopamine. In later tests, these rats also showed fewer drug-seeking behaviors.
The findings suggest genetic changes affect the brain’s reward circuitry and may explain the craving for dopamine stimulating substances during withdrawal. However, it seems to be dopaminylation and not typical dopamine that dictates drug-seeking habits.
Given the vital role of serotonin and dopamine in mental health, the implications will likely go beyond addiction. For instance, if antidepressants work by activating this epigenetic process rather than merely supplying more serotonin, it could explain why the drugs take a while to have an effect.
Takeaways:
- The same enzyme involved in binding serotonin to H3 causes dopamine to attach to H3.
- Histone H3 glutamine 5 dopaminylation (H3Q5dop) plays a critical role in cocaine-induced transcriptional plasticity in the midbrain.
- The study findings establish a neurotransmission-independent role for nuclear dopamine in relapse-related transcriptional plasticity in the VTA.
- In short, this discovery could change the way we view addiction, mental illness, and epigenetics. It could also mean we’re closer to finding treatments that work in the long run.
You can read the original publication of this study here: Dopaminylation of histone H3 in ventral tegmental area regulates cocaine seeking