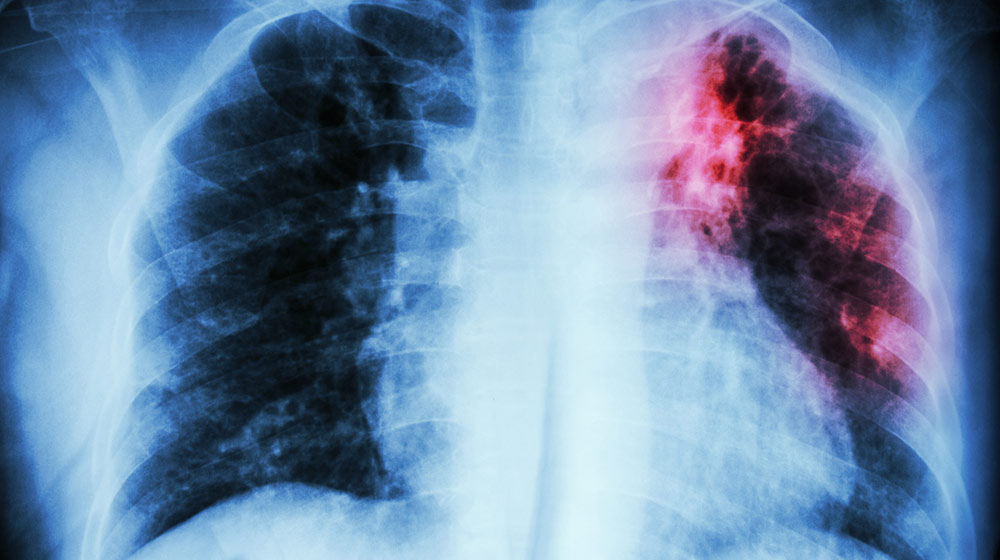
For decades, tuberculosis (TB) has been considered to be one of the deadliest and oldest diseases. Yet, it remains a major threat for many people across the globe. The treatment usually involves using multiple drugs in the course of 6 to 30 months. In some cases, the treatment becomes ineffective due to drug-resistant strains, including antibiotic resistance, which develops during the treatment.
Despite all the advancements and breakthroughs in the medical field in an attempt to get it under control, tuberculosis is still one of the top 10 deadliest infectious illnesses in the world today.
Experts have been unable to explain how this illness, caused by a slow-growing bacterial species, acquires resistance so fast. The pathogen appears to be genetically stable. Nonetheless, it is capable of adjusting to numerous medicines and host immunological responses. To better understand this phenomenon, scientists are going beyond the genetic side of tuberculosis and into epigenetics for solutions.
San Diego State University (SDSU) researchers discovered new evidence implicating epigenetics in TB medication resistance in a paper published in eLife. They focused on DNA methylation as a possible explanation for the phenotypic diversity found in M. tuberculosis, the bacterium that causes tuberculosis.
According to their research, this bacteria has the ability to quickly diversify, resulting in many subpopulations with diverse features or phenotypes. The mechanism is known as intercellular mosaic methylation, and it permits resistant strains to survive. Although antibiotics can kill many species of bacteria, the large number of mutations created in this way makes it impossible to eradicate all variants.
Read the original publication of this study here: [ Epigenetics May Explain How Tuberculosis Develops Antibiotic Resistance ]
This article aims to look into the development of antibiotic resistance to tuberculosis on an epigenetic level

Epigenetics May Explain How Tuberculosis Develops Antibiotic Resistance
In this study, researchers observed how tuberculosis develops resistance to antibiotics and other treatments by extracting samples from 154 tuberculosis patients. The M. tuberculosis and M. africanum samples were prepared and extracted, which are then incubated until fully grown into a bacterial lawn. Later then the DNA is isolated from cells in the stationary growth phase and performs DNA sequencing.
Circularization was then used to confirm a circular genome using amos’ minimus2 or circlator. Genomes failed assembly quality check if they could not be circularized, if consensus polishing resulted in five or more variations after three iterations, or if PBHoney found a structural variant in the assembly supported by at least 10% of the reads.
This integrated study of the DNA adenine methylomes of 93 clinical isolates highlights DNA methylation as a critical source of variation in the MTBC. The findings pave the way for future research into the functions of DNA adenine methylation in M. tuberculosis physiology and adaptive evolution.
The finding of constitutive IMM-driven by MTase genotype, in particular, raises the potential that variations in DNA adenine methylation translate into disparities in adaptive ability across MTBC strains.
The researchers come to the theory by looking at the CT lung scans of the individuals who are “cured” and still show possible bacterial activity. They believe this also explains why diagnostic testing does not anticipate treatment failure in certain patients and why other patients return months later with the illness reemerging in a considerably more resistant condition.
Bacterial infections can significantly influence the host epigenome, making the host more susceptible to disease. Genetic changes frequently cause antibiotic resistance. M. tuberculosis, on the other hand, employs epigenetic processes to adapt to any therapy efficiently.
Not only that, epigenetics has a role in bacterial and host variables that lead to M. tuberculosis infection development. For years, the researchers of this study have been researching epigenetics in tuberculosis. They found that bacterial epigenetics may affect gene expression, resulting in a diversity of phenotypes even when the genotypes are similar.
By cooperating with other researchers from across the world to evaluate drug-resistant samples from hundreds of TB patients in China, India, Europe, the Philippines, and South Africa, they discovered that some of the samples included mutations that resulted in varying DNA methylation, which have a lot more variety in their epigenome.
Surprisingly, no consistent patterns were identified, and methylation was fairly haphazard. As a result, the researchers used the most recent genomic and epigenomic technologies to describe and analyze variations among cells within a colony from a single isolate or person. Because the reference genome lacked a common structure, they rebuilt each individual genome and assessed its epigenetic markers.
This enabled them to discover even the most minor changes that may potentially affect gene expression.
Takeaways
- Even though current TB treatments efficiently prevent and kill bacterial growth, they do not stop intercellular mosaic methylation.
- Hopefully, focusing on this newly discovered diversification process would prevent short-term epigenetic resistance, killing bacteria before they change in the genome and develop long-term resistance.
- Now that we have data linking epigenetics to TB’s persistence, perhaps we can use this knowledge to develop treatment and preventative strategies that will ultimately eliminate this extremely contagious disease for good.