One animal study shows a link between telomere length and metabolic aging. Read on to learn all about it.
RELATED: How To Turn Back Your Biological Clock? Know Your Epigenetic Biomarkers
In this article:
- What Are Telomeres?
- How Are They Linked to the Lifespan?
- Are there Ways to Lengthen Telomeres?
- What Happens When You Lengthen Telomeres in Mice?
Telomere Length and Metabolic Aging: What You Need to Know
What Are Telomeres?
Telomeres are protein structures at the end of chromosomes. They serve to protect the ends of chromones to prevent DNA damage.
They also play a role in DNA repair activities and help maintain chromosome stability. Unfortunately, telomeres will shorten with every cell division.
How Are They Linked to the Lifespan?

At a certain point, telomeres become too short to support cell division. When this happens, cells begin to age and, eventually, die.
Aside from tissue aging, shorter telomere length is also linked to a variety of diseases such as:
- Type 2 diabetes
- Various cancers
- Neurological diseases
- Cardiovascular diseases
They believe that short telomeres may be one of the triggers of these diseases. It’s not just the length of telomeres that matters, but also the rate they are diminishing.
Researchers link the speed of telomere shortening to the lifespan of a species. For example, humans have shorter telomeres than mice, but mice telomeres get shorter a lot quicker. So mice have a shorter lifespan compared to humans.
RELATED: Live Longer: The Secret Is Hidden In Your Own Body
Are there Ways to Lengthen Telomeres?
In 2009, three scientists won the Nobel Prize for discovering telomerase.
What is telomerase? It’s an enzyme that helps synthesize telomeres.
There are some promising results from clinical studies with humans, but these studies are limited to a telomerase activator supplement’s effects. With animal studies, researchers can generate mice with longer telomeres by culturing embryonic cells.
They then track the development of the mice through their lifespan to determine the effects of longer telomeres. A team of researchers ran this animal study and published their surprising results in the journal Nature Communication.
What Happens When You Lengthen Telomeres in Mice?
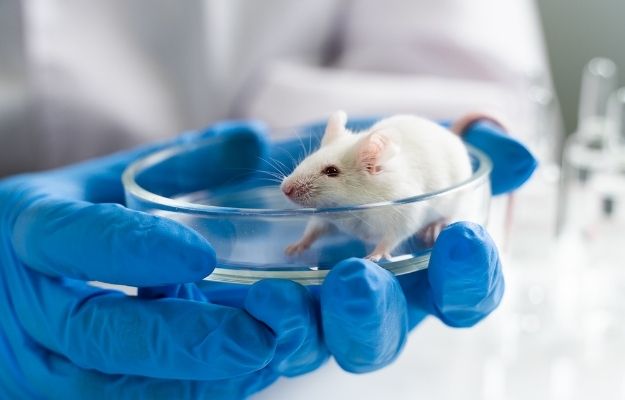
Without manipulating their genetics, researchers generated mice with hyper-long telomeres. They compared the development of these mice to a control group of unaltered mice.
Here are the researcher’s main findings:
- Hyper-long telomere mice maintained their longer telomeres up to old age. They also had normal tissue up to their old age.
- Hyper-long telomere mice were leaner than the control group throughout their lifespan. They had less fat accumulation. Apart from this, there were no other overtly physical differences between the two groups.
- Hyper-long telomere mice had better metabolic parameters. They had lower levels of “bad” cholesterol, better insulin tolerance, and lower levels of alanine aminotransferase (ALT) in their blood. An excess of the ALT enzyme causes liver problems.
- Hyper-long telomere mice lived longer than the control group. They also had fewer cases of spontaneous tumor growth.
- Hyper-long telomere mice have normal cognitive abilities, mouse coordination, and sensory perception. They did not do significantly worse or better in various cognitive tests compared to the control group.
- Hyper-long telomere mice did not exhibit unusual telomerase expression.
- There were less DNA damage and cellular senescence in hyper-long telomere mice. Cellular senescence is a state of stable cell arrest and another biomarker of aging.
- There was an increase in mitochondrial function in the cells of hyper-long telomere mice.
The results of this study imply that long telomeres in mice may be beneficial for their species. Mice with longer telomeres experience a delay in metabolic aging and, in turn, have healthier and longer lives.
It’s also important to point out that lengthening telomeres did not seem to increase the risk of cancer in mice. Older studies suggest that longer telomeres in humans may increase the risk of cancer.
The researchers believe that these promising results show that natural selection favors species with longer telomeres. Elongating telomeres at the embryonic stage may be a possible mechanism for lifespan expansion in the future.
The study results are quite promising, but it’ll take some time before experiments like this can be translated into human clinical trials. In the meantime, there are other aging biomarkers you can explore.
For example, a biological age test can measure DNA methylation levels across the genome. If you’re interested in learning more about this, visit the TruDiagnostic website.
Do you think having longer telomeres would be beneficial for humans too? Please share your thoughts with us in the comments section below.
Up Next: